OUR QUALITY VISION
- From the very inception of our Indian pharmaceutical company, quality is the core of whatever we do. Thus, we have been persistently putting all over efforts in creating Our Quality Vision health care products for people.
- The patient's well-being as well as the reliance upon reliable and trustworthy suppliers serve as the cornerstone of our organization. We are committed to harnessing all available resources and expertise for ensuring the best practices to produce high-quality, consistent, safe healthcare products. The leadership of our organization is dedicated to extending all the resources for implementing the most effective quality management system, with continuous improvement, meeting regulatory compliance, and quality policy principles, and identifying all opportunities for continuous improvement.
Our quality practices
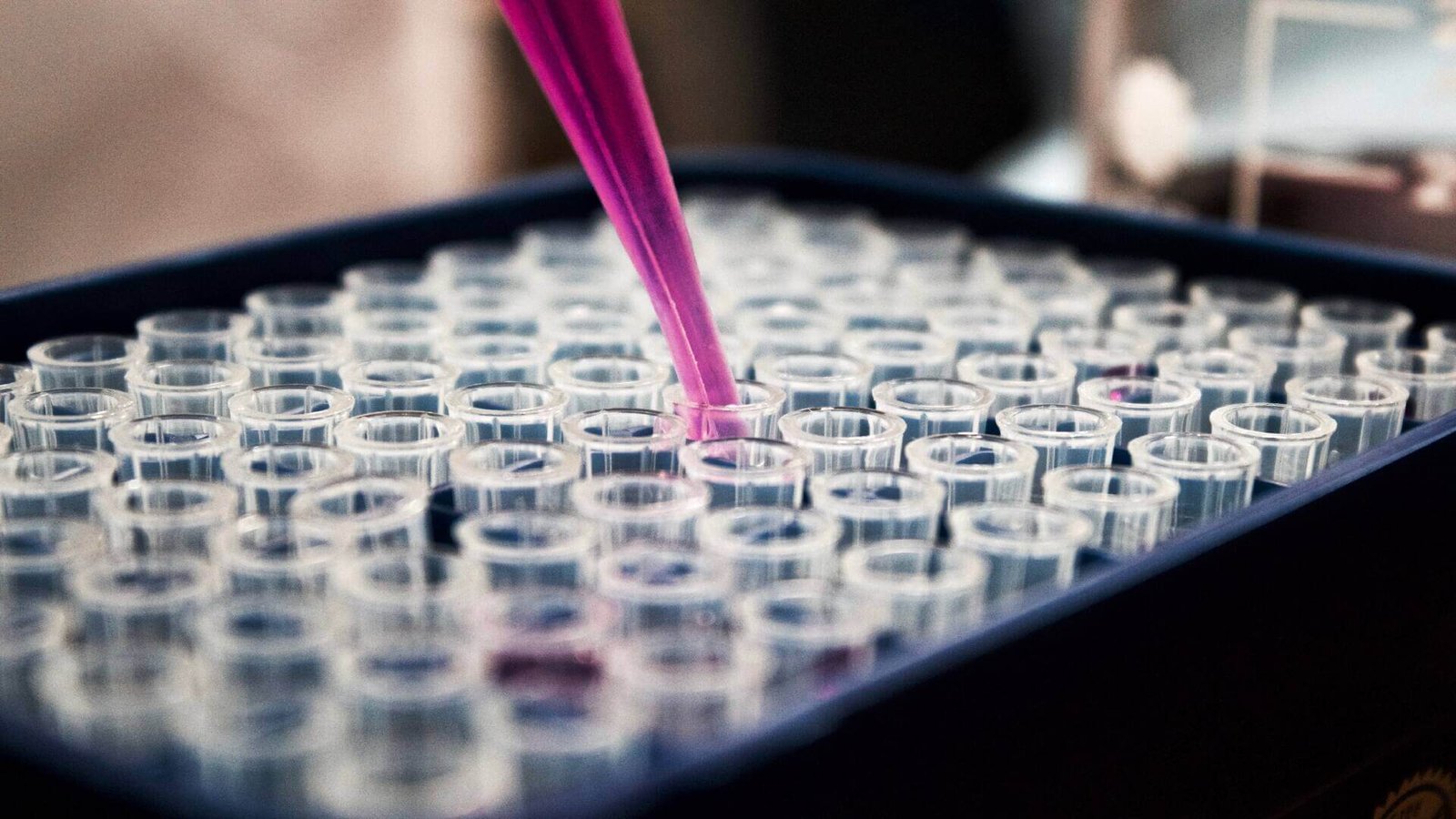
- At Gleason Health Care, we strongly believe that quality and safety cannot actually be integrated into the products solely on the manufacturing line. But the quality and safety standards are initiated through the most appropriate sourcing, testing of raw material, continuing through the testing and reviewing of the finished products.
- Through the administration of stringent & comprehensive regulatory control, we ensure the delivery of therapeutic products, conforming to the highest quality and safety standards. Through regular auditing of all systems, processes, facilities, and suppliers, we can maintain the highest quality standards throughout the stages of each product manufacturing cycle.

Quality Management System
- Since healthcare products are directly related to the lives and health of human beings and thus, the quality of these products must be guaranteed. Due to this, our pharmaceutical company puts constant efforts to secure the utmost quality of all products through compliance with Goods Manufacturing Practice (GMP) for health care outcomes.
- Our quality management & compliance is integral to all aspects of our product life cycle, ranging from innovation, and manufacturing to commercialization. Our quality management system stands at the forefront of the production and distribution processes within the industry.
- Our quality management system involves continuous monitoring as well as the implementation of the regulatory trends within the industry, regular reviewing of processes, and products monitoring key quality indicators as described in our manual.

Global Quality Metrics
Quality metrics play a crucial role in the drug manufacturing industry for driving continuous improvement efforts while monitoring quality systems as well as processes. The global quality metrics are systematic and serve as the most refined representation of all our manufacturing operations. Since the quality is measured at distinctive levels in many different processes and thus, Global Quality Metrics enable us to reach towards highest quality performance. Due to this, our organization will benefit from continuous improvement in operating performance and GMP compliance.
Quality Data Governance
- Data governance is vital for compliance with existing regulatory expectations as one of the main quality system policies. The quality data governance policy is serving as the crux and integrated part of the Quality Management System (QMS).
- The robust data governance, as well as the quality system that we adhere to, is not only paving the way towards seamless patient care but also acts as the key driver in generating explicable outcomes for all patients, and experiences as well as improving our productivity.
